Preparation of galactose oxidase functional phosphorescent quantum dots and detection of D-galactose.,Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - X-MOL
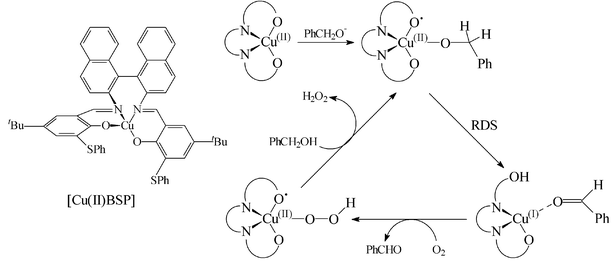
Cu( ii )-nitroxyl radicals as catalytic galactose oxidase mimics - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/B305941C

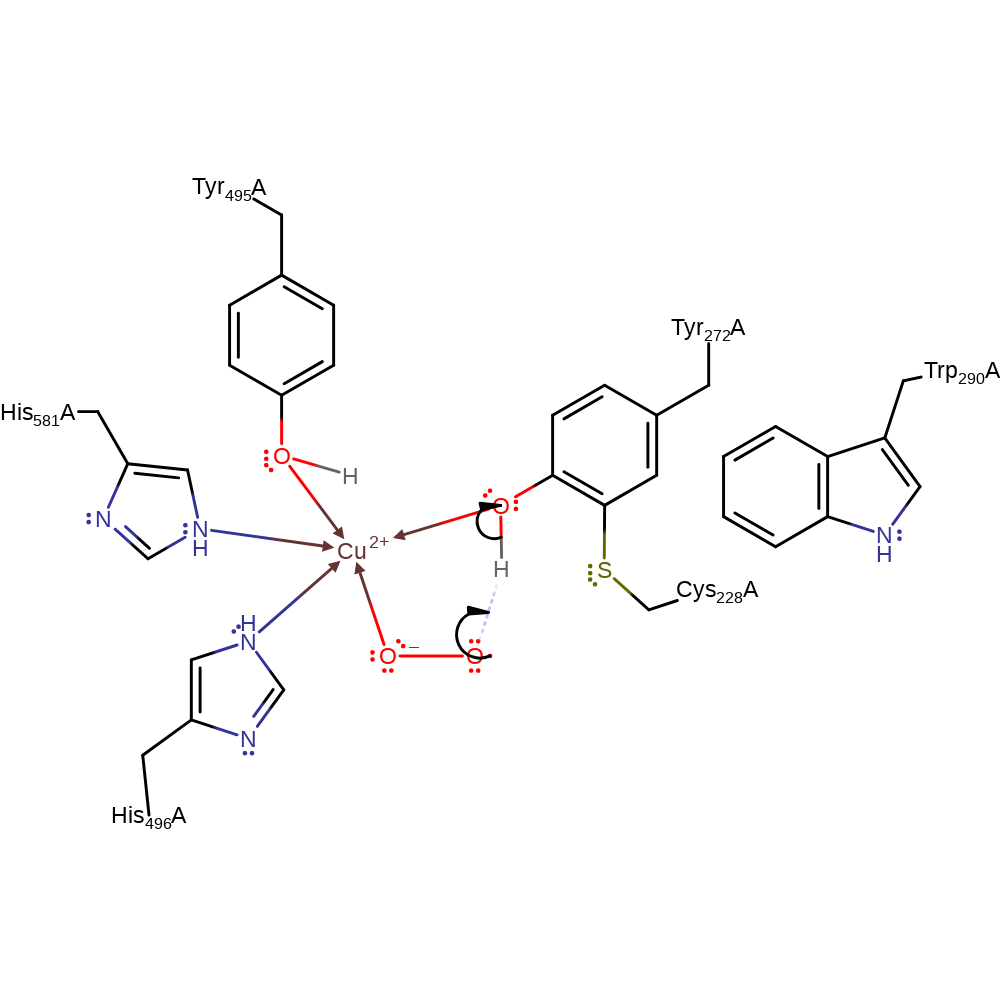
Cu(I)-dependent Biogenesis of the Galactose Oxidase Redox Cofactor* - Journal of Biological Chemistry

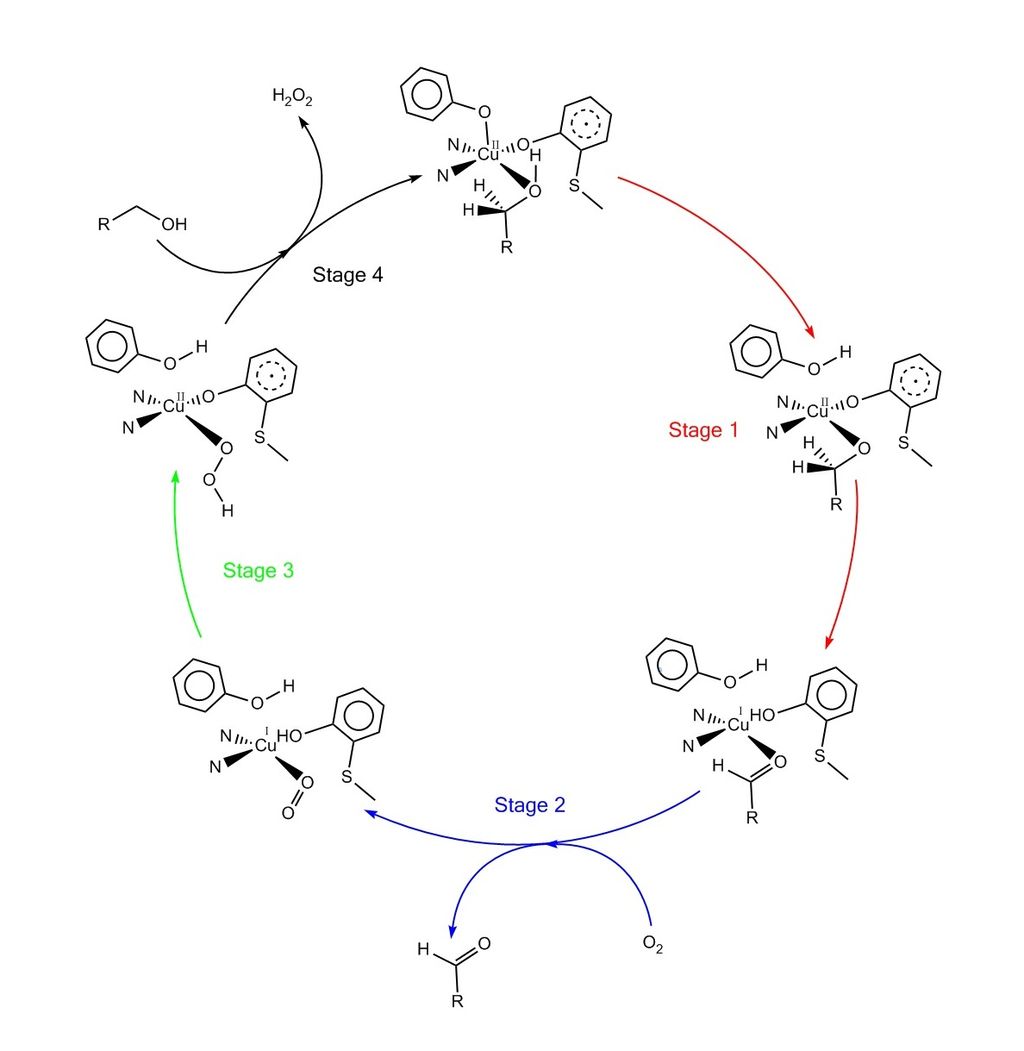
Catalytic Promiscuity of Galactose Oxidase: A Mild Synthesis of Nitriles from Alcohols, Air, and Ammonia - Vilím - 2018 - Angewandte Chemie - Wiley Online Library

Recent Advances in One‐Electron‐Oxidized CuII–Diphenoxide Complexes as Models of Galactose Oxidase: Importance of the Structural Flexibility in the Active Site - Oshita - 2020 - Chemistry – A European Journal - Wiley Online Library
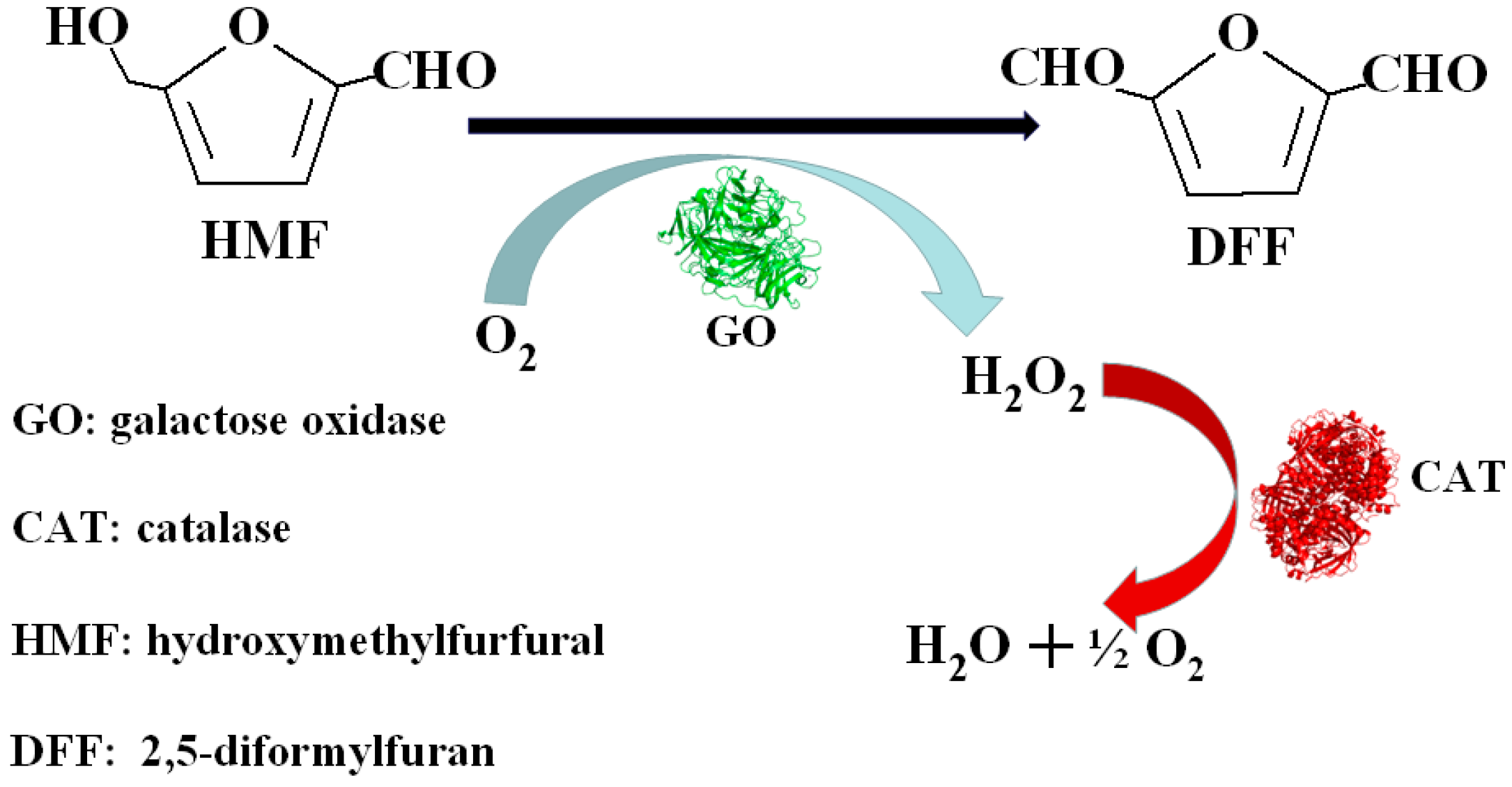
Molecules | Free Full-Text | Co-Immobilization of Tri-Enzymes for the Conversion of Hydroxymethylfurfural to 2,5-Diformylfuran | HTML
Redox active ligand and metal cooperation for C(sp2)–H oxidation: extension of the galactose oxidase mechanism in water-mediated amide formation - Dalton Transactions (RSC Publishing)