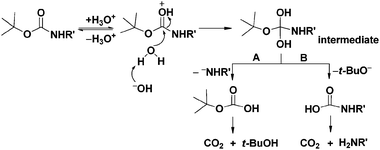
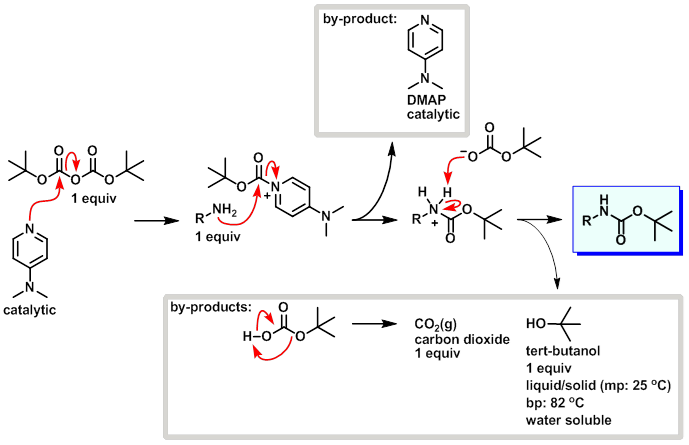
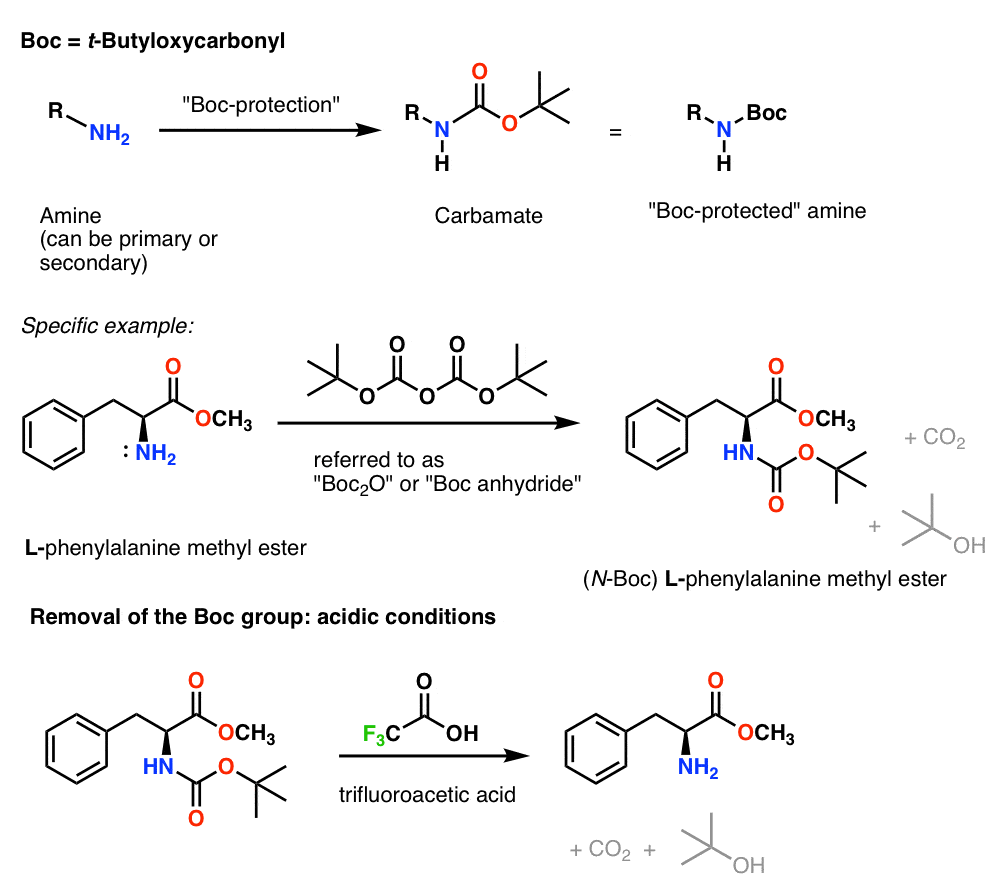
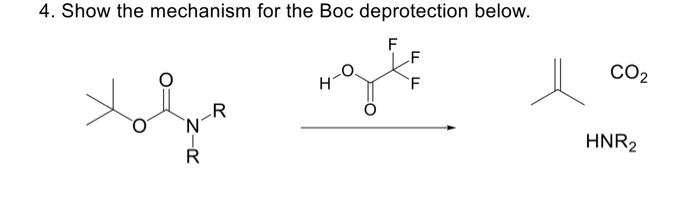
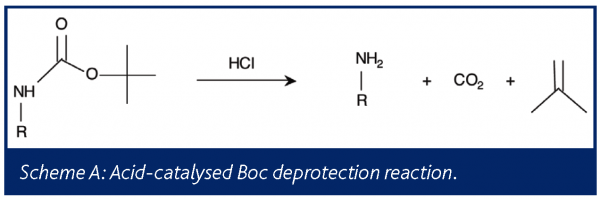
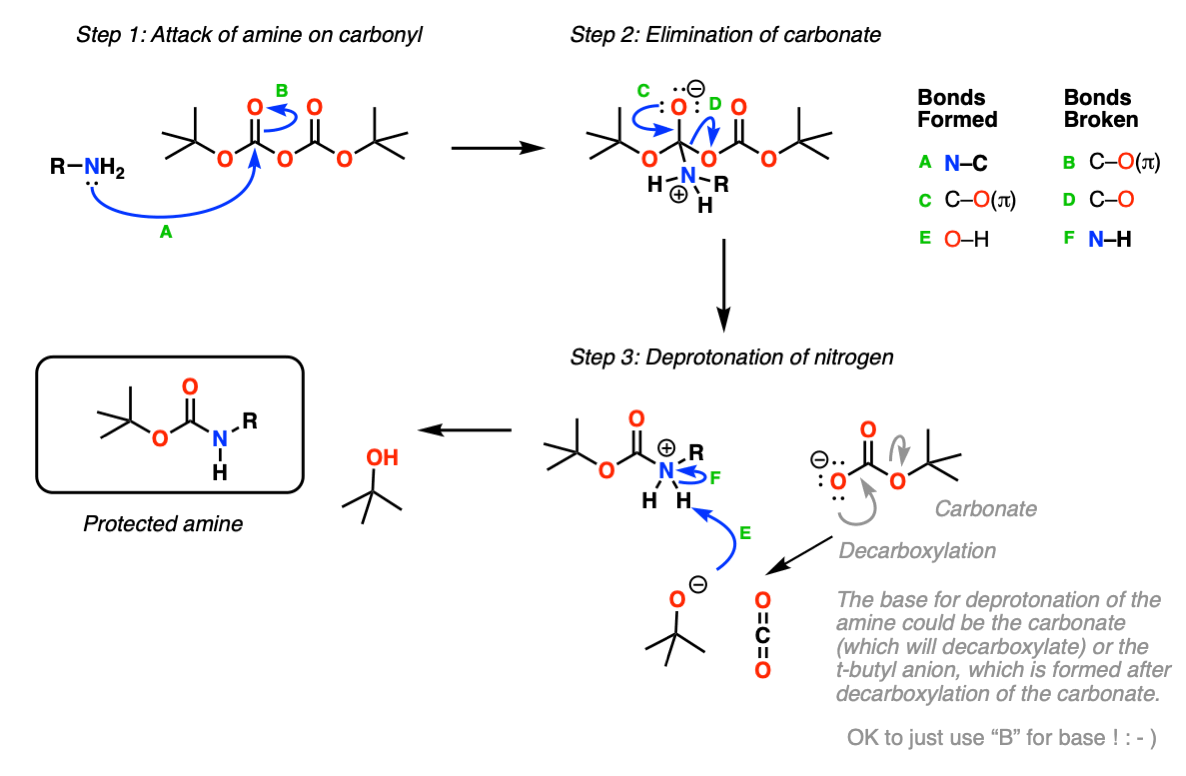
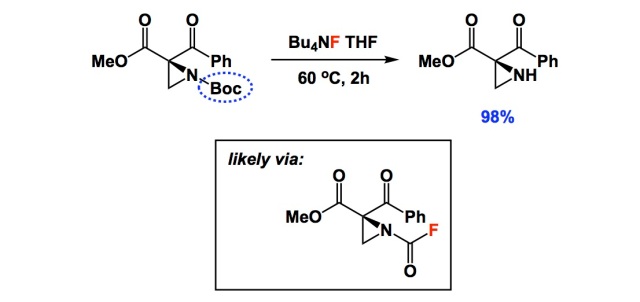
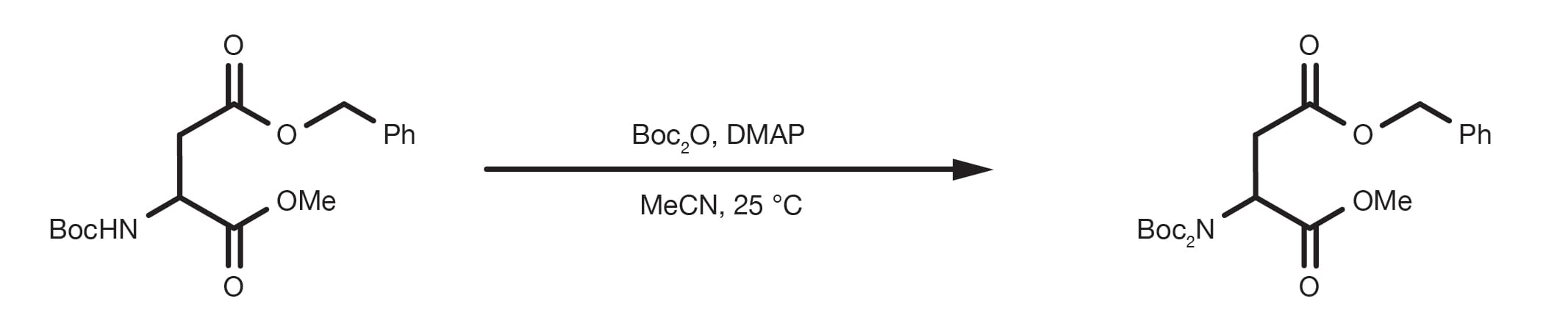
ChemSpider SyntheticPages | BOC deprotection of an aminophenylethyl methanesulfonate using hydrochloric acid

organic chemistry - Mechanism for cyclic enamine formation after N-Boc deprotection - Chemistry Stack Exchange

Deprotection of N‐tert‐Butoxycarbonyl (Boc) Protected Functionalized Heteroarenes via Addition–Elimination with 3‐Methoxypropylamine - Gulledge - 2020 - European Journal of Organic Chemistry - Wiley Online Library

Suppression of Side Reactions During Final Deprotection Employing a Strong Acid in Boc Chemistry: Regeneration of Methionyl Residues from Their Sulfonium Salts | Semantic Scholar

organic chemistry - Deprotection of Boc using TAF to obtained free amine group - Chemistry Stack Exchange