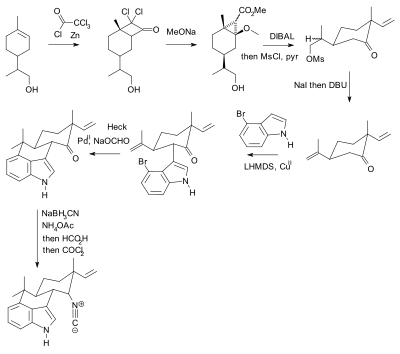
Scheme 7 Synthesis of neurodazine. Reagents and conditions: a) benzyl... | Download Scientific Diagram

Sonochemical protocol for protection and deprotection of functional groups in organic synthesis - ScienceDirect

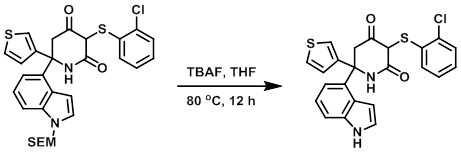
Acid-mediated cyclizations of SEM-protected heterocyclic anilines and adjacent hydroxyls or enol-ethers - ScienceDirect

PDF) An Efficient Deprotection of N -Trimethylsilylethoxymethyl (SEM) Groups From Dinucleosides and Dinucleotides

Scheme 7 Synthesis of neurodazine. Reagents and conditions: a) benzyl... | Download Scientific Diagram

Optimisation of a key cross-coupling reaction towards the synthesis of a promising antileishmanial compound. - Abstract - Europe PMC

Synthesis of Raputimonoindoles A–C and Congeners - Kock - 2019 - European Journal of Organic Chemistry - Wiley Online Library
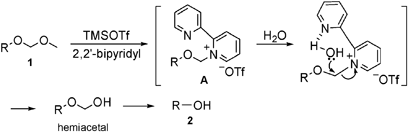
Remarkable effect of 2,2′-bipyridyl : mild and highly chemoselective deprotection of methoxymethyl (MOM) ethers in combination with TMSOTf (TESOTf)–2, ... - Chemical Communications (RSC Publishing) DOI:10.1039/B907910F

Synthesis of Raputimonoindoles A–C and Congeners - Kock - 2019 - European Journal of Organic Chemistry - Wiley Online Library

Synthesis of Raputimonoindoles A–C and Congeners - Kock - 2019 - European Journal of Organic Chemistry - Wiley Online Library

C-H bonds as ubiquitous functionality: a general approach to complex arylated imidazoles via regioselective sequential arylation of all three C-H bonds and regioselective N-alkylation enabled by SEM-group transposition. - Abstract - Europe

Sonochemical protocol for protection and deprotection of functional groups in organic synthesis - ScienceDirect

Acid-mediated cyclizations of SEM-protected heterocyclic anilines and adjacent hydroxyls or enol-ethers - ScienceDirect
![Molecules | Free Full-Text | Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization | HTML Molecules | Free Full-Text | Synthesis of New 5-Aryl-benzo[f][1,7]naphthyridines via a Cascade Process (Ugi-3CR/Intramolecular Aza-Diels-Alder Cycloaddition)/Aromatization | HTML](https://www.mdpi.com/molecules/molecules-23-02029/article_deploy/html/images/molecules-23-02029-sch003.png)



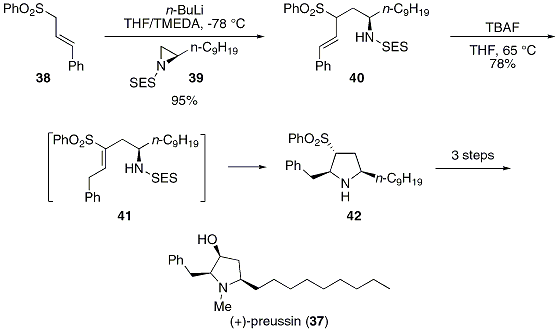
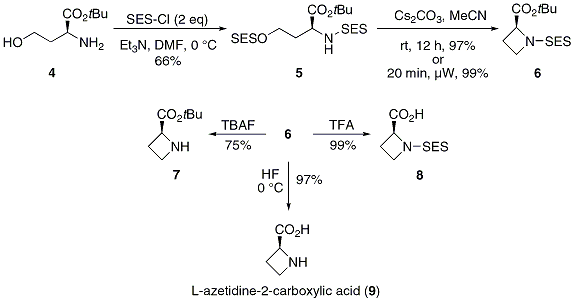

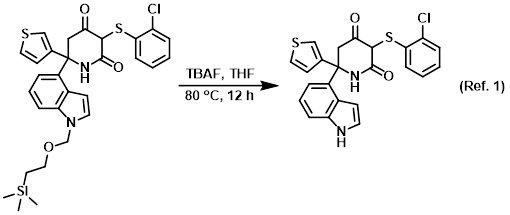
![2-(Trimethylsilyl)ethoxy]methyl acetal (SEM)protecting group. 2-(Trimethylsilyl)ethoxy]methyl acetal (SEM)protecting group.](https://synarchive.com/images/png/45/0000045-02-12b1.png)