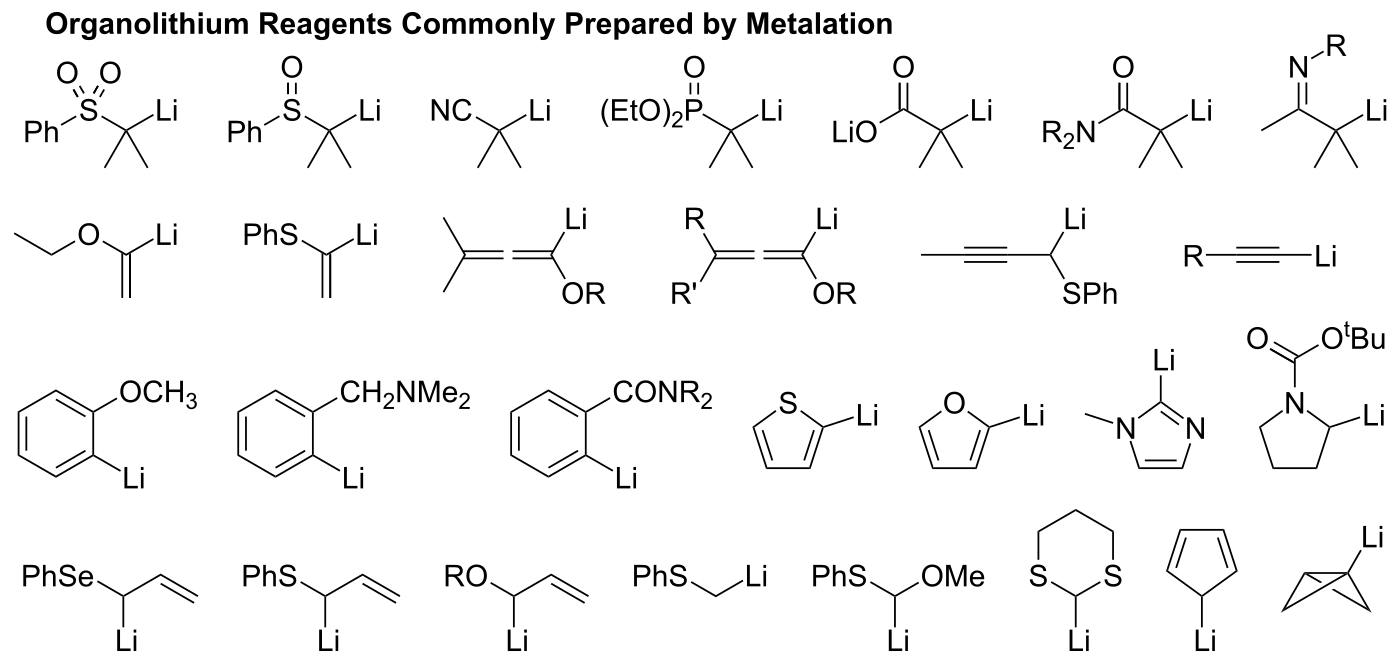
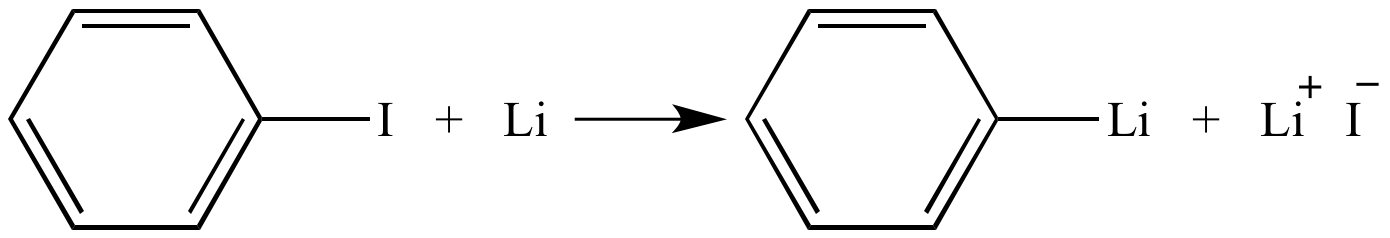
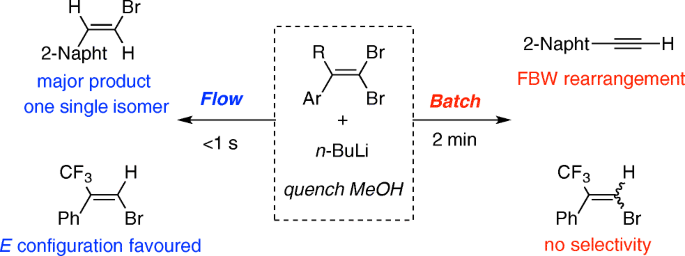
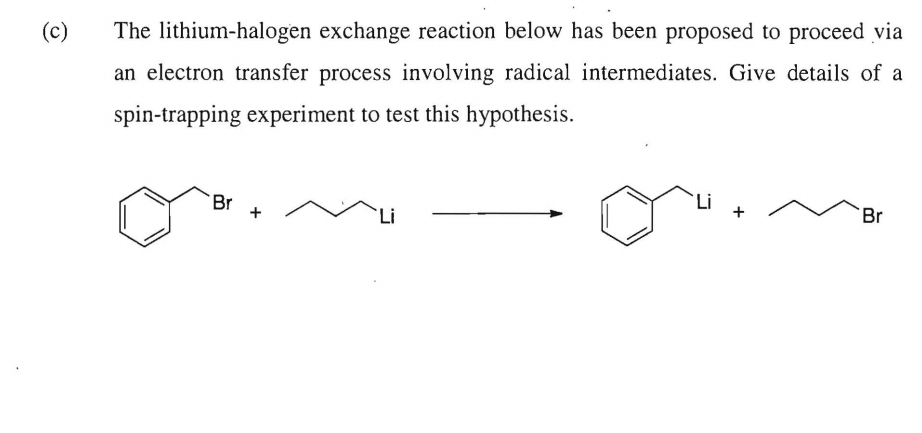
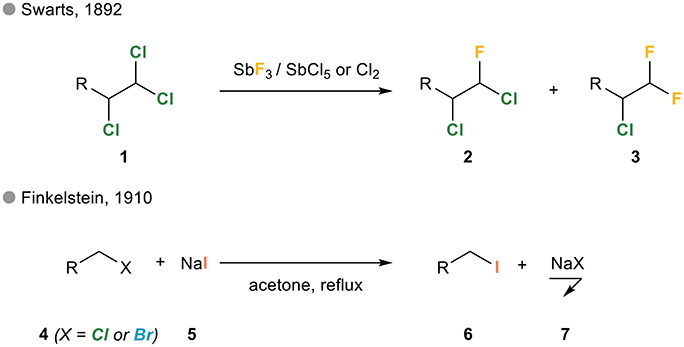
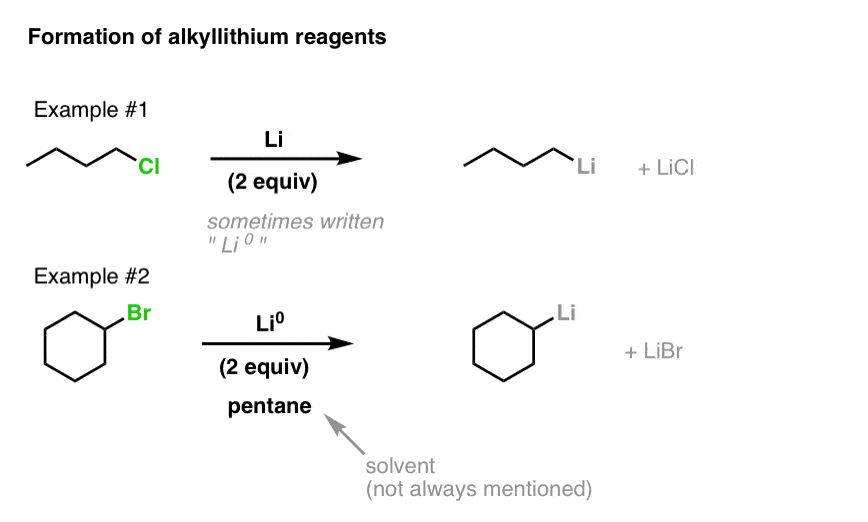
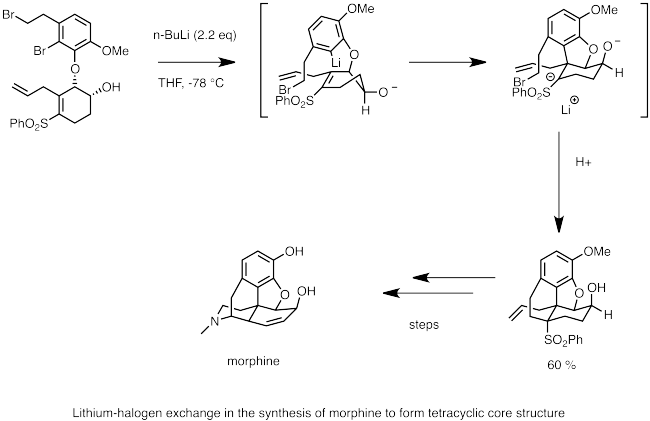
Regioselective lithium–halogen exchange and palladium-catalyzed cross-coupling reactions of 2,4-dihaloquinolines - ScienceDirect
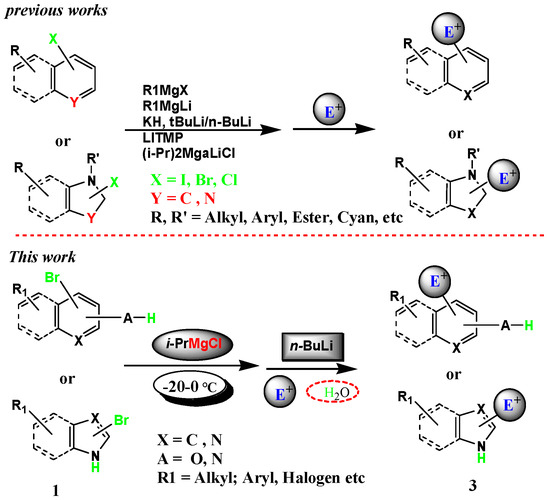
Molecules | Free Full-Text | Halogen–Metal Exchange on Bromoheterocyclics with Substituents Containing an Acidic Proton via Formation of a Magnesium Intermediate | HTML
![Molecules | Free Full-Text | Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans | HTML Molecules | Free Full-Text | Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans | HTML](https://www.mdpi.com/molecules/molecules-20-19449/article_deploy/html/images/molecules-20-19449-g003.png)
Molecules | Free Full-Text | Selective Halogen-Lithium Exchange of 1,2-Dihaloarenes for Successive [2+4] Cycloadditions of Arynes and Isobenzofurans | HTML

Lithium–Bromide Exchange versus Nucleophilic Addition of Schiff's base: Unprecedented Tandem Cyclisation Pathways - Orr - 2019 - Chemistry – A European Journal - Wiley Online Library
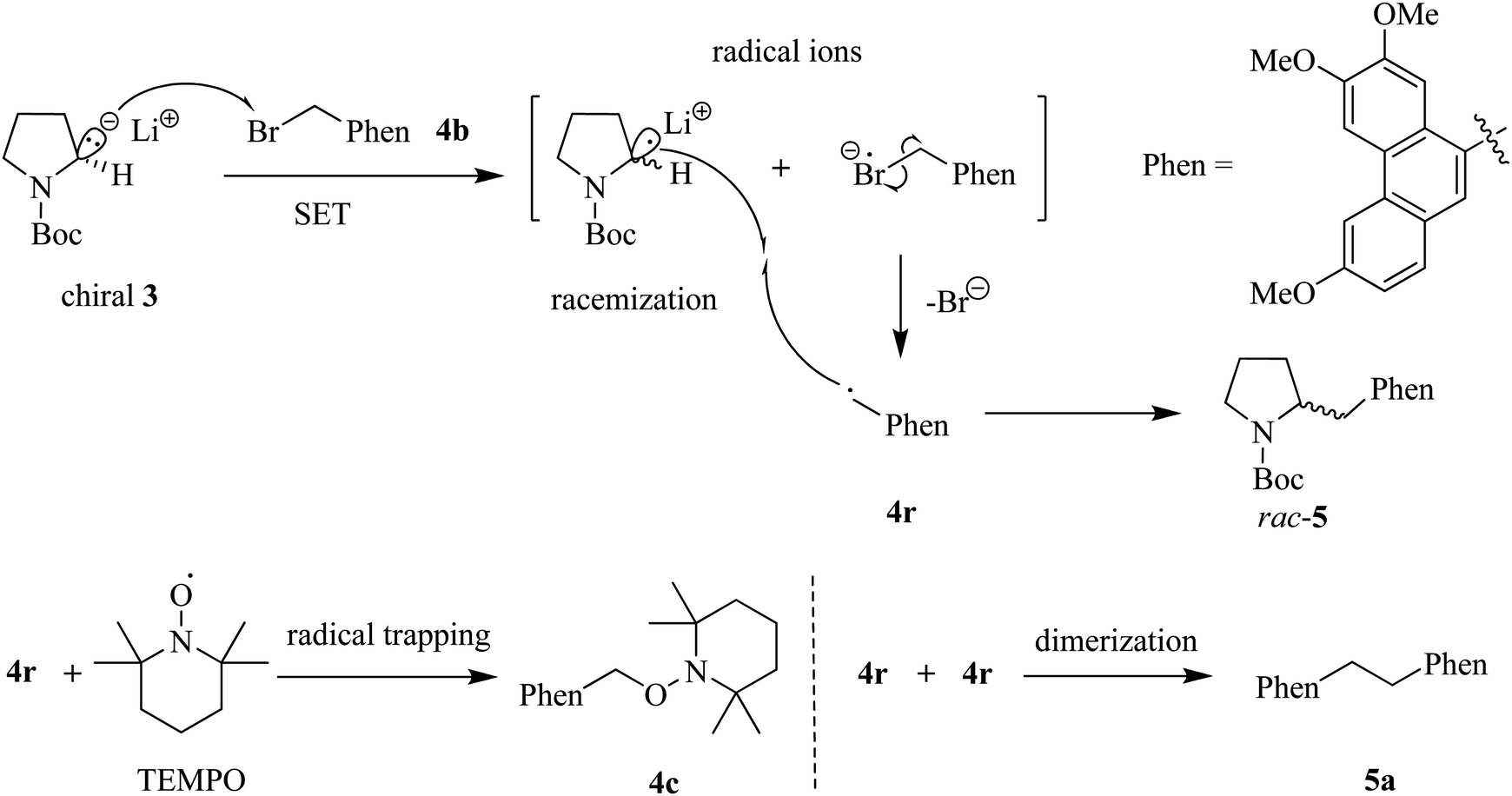
Total synthesis of phenanthroindolizidine alkaloids via asymmetric deprotonation of N -Boc-pyrrolidine - RSC Advances (RSC Publishing) DOI:10.1039/C3RA47465H

Lithium–Bromide Exchange versus Nucleophilic Addition of Schiff's base: Unprecedented Tandem Cyclisation Pathways - Orr - 2019 - Chemistry – A European Journal - Wiley Online Library